Reduction
The Birch reduction is a dissolving metal promoted reaction in which aromatic rings are partially reduced by an alkali metal in liquid ammonia, usually in the presence of a proton source (such as an alcohol). Recently, we have extended and applied this methodology to encompass the partial reduction of a variety of heteroaromatic compounds. These studies have resulted in a high-yielding approach to dihydropyridine, pyrroline, dihydrofuran compounds with varied substitution patterns, all formed directly from electron-deficient pyridines, pyrroles and furans respectively.
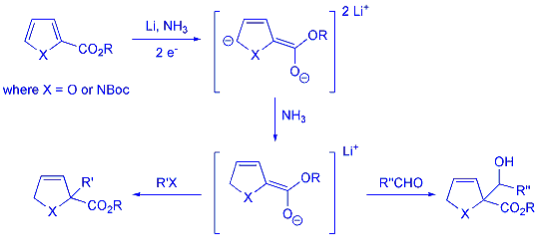
We have expanded the usefulness of the classic Birch reduction through the development of an ammonia-free variant (using di-tert-butyl biphenyl as the electron carrier) allowing us to quench the enolates formed with reactive electrophiles such as enolisable aldehydes, acid chlorides and chloroformates. None of the aforementioned electrophiles are compatible with standard Birch conditions because they react preferentially with the solvent.
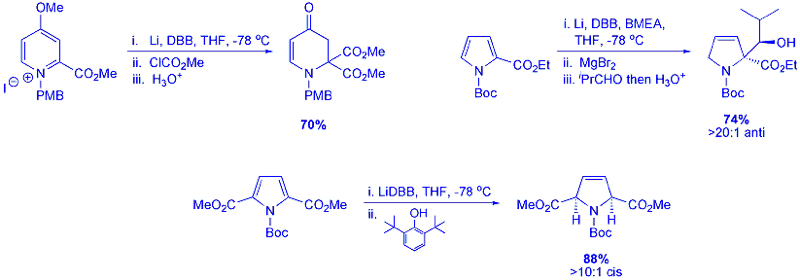
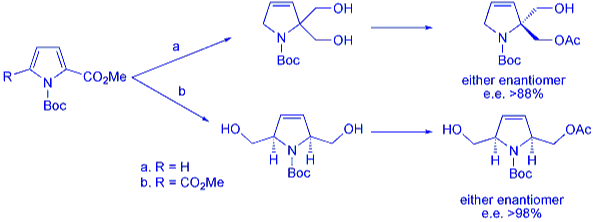
Further investigation has led to the development of enantioselective routes to substituted pyrroline compounds. This has been achieved via a chiral protonation approach using easily accessible chiral acids, such as ephedrine and oxazolidinones, to quench enolates formed during the partial reduction process. Alternatively, enzyme desymmetrisation of symmetrical diol compounds formed from the partial reduction products of substituted pyrroles is also reported. This leads to formation of both enantiomers of 2,2- and 2,5-disubstituted N-Boc pyrrolines in excellent e.e. and yields.
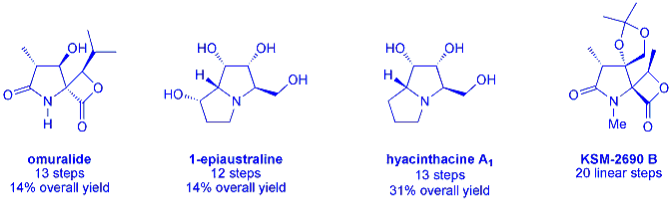
Our methodology has been proven in the syntheses of numerous complex natural product targets including 1-epiaustraline, hyacinthcine A1, omuralide and KSM-2690 B. Current targets include cylindricine A and members of the hyacinthacine family of compounds.