Publications
Books
Oxidation and Reduction in Organic Synthesis: OUP Chemistry Primer No. 6
Timothy J. Donohoe
Oxford University Press, 2000, ISBN 0198556640.
Oxidation and Reduction in Organic Synthesis: OUP Chemistry Primer No. 6
Timothy J. Donohoe
Oxford University Press, 2000, ISBN 0198556640.
Independent Publications
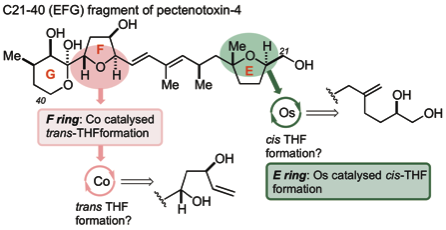
187 Cobalt versus osmium: control of both trans and cis selectivity in construction of the EFG rings of pectenotoxin 4
Ahria Roushanbakhti, Yifan Liu, Paul C. M. Winship, Michael J. Tucker, Wasim M. Akhtar, Daryl S. Walter, Gail Wrigley and Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2017, in the press
Ahria Roushanbakhti, Yifan Liu, Paul C. M. Winship, Michael J. Tucker, Wasim M. Akhtar, Daryl S. Walter, Gail Wrigley and Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2017, in the press
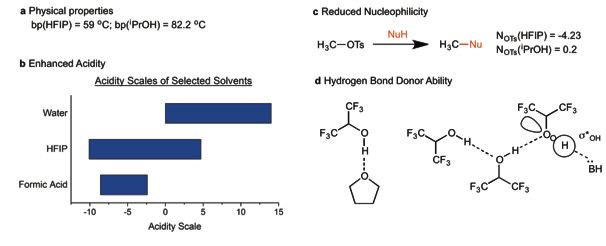
186 Hexafluoroisopropanol as a highly versatile solvent
Ignacio Colomer, Anna E. R. Chamberlain, Maxwell B. Haughey and Timothy J. Donohoe
Nature Reviews Chemistry, 2017, in the press
Ignacio Colomer, Anna E. R. Chamberlain, Maxwell B. Haughey and Timothy J. Donohoe
Nature Reviews Chemistry, 2017, in the press
185 Pyruvate Enolate Arylation and Alkylation: OBO Ester Protected Pyruvates as Useful Reagents in Organic Synthesis
Carlos Henrique Esteves, Christopher Hall, Peter Smith and Timothy J. Donohoe
Organic Letters, 2017, in the press
Carlos Henrique Esteves, Christopher Hall, Peter Smith and Timothy J. Donohoe
Organic Letters, 2017, in the press

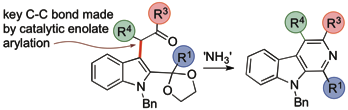
184 Catalytic enolate arylation with 3-bromoindoles allows the formation of beta-carbolines
C. Henrique Estavez Alves, Peter D. Smith, and Timothy J. Donohoe
J. Org. Chem. 2017, 82, 4435-4443
DOI: 10.1021/acs.joc.7b00299
C. Henrique Estavez Alves, Peter D. Smith, and Timothy J. Donohoe
J. Org. Chem. 2017, 82, 4435-4443
DOI: 10.1021/acs.joc.7b00299
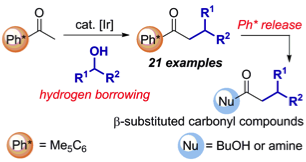
183 Hydrogen Borrowing Catalysis with Secondary Alcohols: A New Route for the Generation of β-Branched Carbonyl Compounds
Wasim M. Akhtar, Choon Boon Cheong, James R. Frost, Kirsten E. Christensen, Neil G. Stevenson, and Timothy J. Donohoe
J. Am. Chem. Soc. 2017, 139, 2577-2580
DOI: 10.1021/jacs.6b12840
Wasim M. Akhtar, Choon Boon Cheong, James R. Frost, Kirsten E. Christensen, Neil G. Stevenson, and Timothy J. Donohoe
J. Am. Chem. Soc. 2017, 139, 2577-2580
DOI: 10.1021/jacs.6b12840
182 Diastereoselective synthesis of the 5-hydroxy-pyrrolidinone amino acid of the microsclerodermins and model studies for an end-game strategy for microsclerodermin B
Christian Winter, Robert D. C. Pullin and Timothy J. Donohoe
Tetrahedron Letters, 2017, 58, 602-605
DOI: 10.1016/j.tetlet.2016.12.045
Christian Winter, Robert D. C. Pullin and Timothy J. Donohoe
Tetrahedron Letters, 2017, 58, 602-605
DOI: 10.1016/j.tetlet.2016.12.045
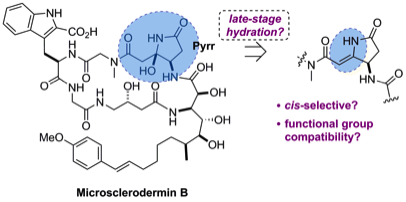
181 Orthogonally protected 1,2 diols from electron-rich alkenes using metal free olefin syn dihydroxylation
Ignacio Colomer, Rosimeire Coura Barcelos, Kirsten E. Christensen, Timothy J. Dnohoe
Organic Letters, 2016, 18, 5880-5883.
DOI: 10.1021/acs.orglett.6b02959
Ignacio Colomer, Rosimeire Coura Barcelos, Kirsten E. Christensen, Timothy J. Dnohoe
Organic Letters, 2016, 18, 5880-5883.
DOI: 10.1021/acs.orglett.6b02959
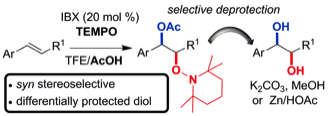
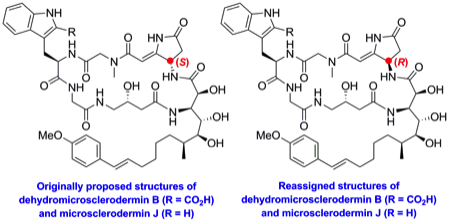
180 Dehydromicrosclerodermin B and microsclerodermin J: total synthesis and structural revision
Ekaterina Y. Melikhova, Robert D. C. Pullin, Christian Winter, Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2016, 55, 9753-9757.
DOI: 10.1002/ange.201604764
Ekaterina Y. Melikhova, Robert D. C. Pullin, Christian Winter, Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2016, 55, 9753-9757.
DOI: 10.1002/ange.201604764
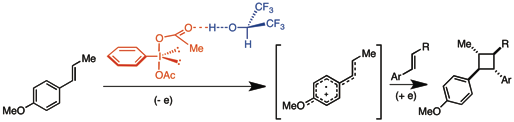
179 Hydrogen bonding to hexafluoroisopropanol controls the oxidative strength of hypervalent iodine reagents
Ignacio Colomer, Christopher Batchelor-McAuley, Barbara Odell, Timothy J. Donohoe, Richard Compton
J. Am. Chem. Soc. 2016,138, 8855-8861.
DOI: 10.1021/jacs.6b04057
Ignacio Colomer, Christopher Batchelor-McAuley, Barbara Odell, Timothy J. Donohoe, Richard Compton
J. Am. Chem. Soc. 2016,138, 8855-8861.
DOI: 10.1021/jacs.6b04057
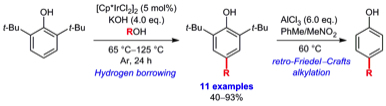
178 Iridium-Catalyzed C4-Alkylation of 2,6-Di-tert-butylphenol using Hydrogen Borrowing Catalysis
James R. Frost, Choon Boon Cheong, Timothy J. Donohoe
Synthesis, 2016, 49, 910-916.
DOI: 10.1055/s-0035-1561439
James R. Frost, Choon Boon Cheong, Timothy J. Donohoe
Synthesis, 2016, 49, 910-916.
DOI: 10.1055/s-0035-1561439
177 Synthesis of aromatic heterocycles using ring-closing metathesis
Harish K. Potukuchi, Ignacio Colomer, Timothy J. Donohoe
Advances in Heterocyclic Chemistry: Alan Katritzky Tribute volume, Chapter 15, 2016, Elsevier, 43-65.
Harish K. Potukuchi, Ignacio Colomer, Timothy J. Donohoe
Advances in Heterocyclic Chemistry: Alan Katritzky Tribute volume, Chapter 15, 2016, Elsevier, 43-65.
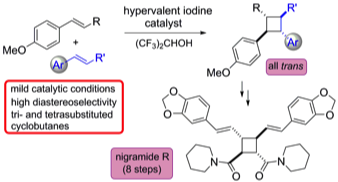
176 Catalytic hypervalent iodine promoters allow styrene dimerisation and the formation of tri- and tetrasubstituted cyclobutanes
Ignacio Colomer, Rosimeire Coura Barcelos and Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2016, 55, 4748-4752.
DOI: 10.1002/anie.201511683
Ignacio Colomer, Rosimeire Coura Barcelos and Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2016, 55, 4748-4752.
DOI: 10.1002/anie.201511683
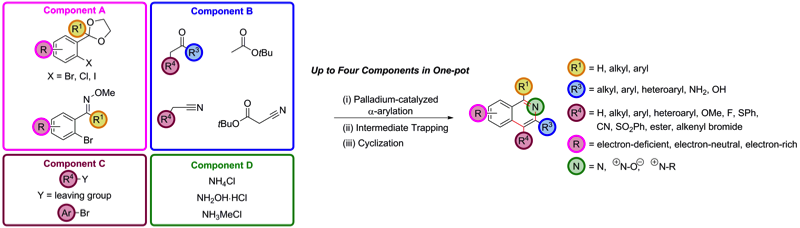
175 Palladium-catalysed enolate arylation as a key C-C bond forming reaction for the synthesis of isoquinolines
Ben S. Pilgrim, Alice E. Gatland, Carlos H. A. Estaves, Charlie T. McTernan, Geraint R. Jones, Matthew R. Tatton, Panayiotis A. Procopiou, Timothy J. Donohoe
Organic and Biomolecular Chemistry, 2016, 14, 1065-1090
Designated as a HOT article
DOI: 10.1039/C5OB02320C
Ben S. Pilgrim, Alice E. Gatland, Carlos H. A. Estaves, Charlie T. McTernan, Geraint R. Jones, Matthew R. Tatton, Panayiotis A. Procopiou, Timothy J. Donohoe
Organic and Biomolecular Chemistry, 2016, 14, 1065-1090
Designated as a HOT article
DOI: 10.1039/C5OB02320C
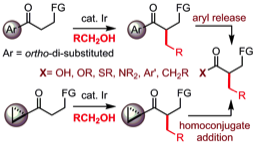
174 Strategic application and transformation of ortho-di-substituted phenyl and cyclopropyl ketones to expand the scope of hydrogen borrowing catalysis
James R. Frost, Choon Boon Cheong, Wasim M. Akhtar, Dimitri F. J. Caputo, Neil G. Stevenson and Timothy J. Donohoe
J. Am. Chem. Soc. 2015, 137, 15664-15667
DOI: 10.1021/jacs.5b11196
James R. Frost, Choon Boon Cheong, Wasim M. Akhtar, Dimitri F. J. Caputo, Neil G. Stevenson and Timothy J. Donohoe
J. Am. Chem. Soc. 2015, 137, 15664-15667
DOI: 10.1021/jacs.5b11196
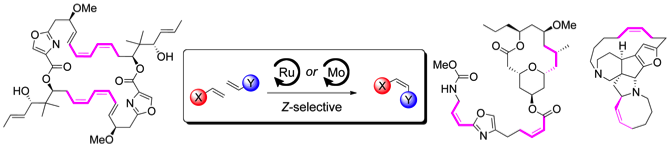
173 Application of catalytic Z-selective olefin metathesis in natural product synthesis
Simon Werrel, Johannes C. L. Walker and Timothy J. Donohoe
Tetrahedron Letters, 2015, 56, 5261-5268
DOI: 10.1016/j.tetlet.2015.07.008
Simon Werrel, Johannes C. L. Walker and Timothy J. Donohoe
Tetrahedron Letters, 2015, 56, 5261-5268
DOI: 10.1016/j.tetlet.2015.07.008
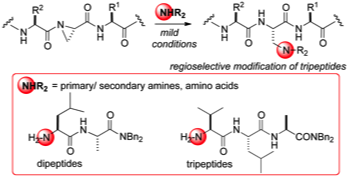
172 Aziridine electrophiles in the functionalisation of peptide chains with amine nucleophiles
Anatol P. Spork and Timothy J. Donohoe
Organic and Biomolecular Chemistry, 2015, 13, 8545-8549
DOI: 10.1039/C5OB00856E
Anatol P. Spork and Timothy J. Donohoe
Organic and Biomolecular Chemistry, 2015, 13, 8545-8549
DOI: 10.1039/C5OB00856E
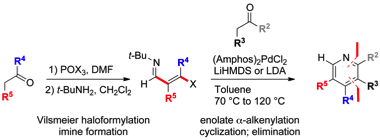
171 Modular Synthesis of Highly Substituted Pyridines via Enolate α-Alkenylation
Leo A. Hardegger, Jacqueline Habegger and Timothy J. Donohoe
Organic Letters, 2015, 17, 3222-3225
DOI: 10.1021/acs.orglett.5b01312
Leo A. Hardegger, Jacqueline Habegger and Timothy J. Donohoe
Organic Letters, 2015, 17, 3222-3225
DOI: 10.1021/acs.orglett.5b01312
170 Palladium-catalyzed alpha-arylation of carbonyls in the de novo synthesis of aromatic heterocycles
Harish K. Potukuchi, Anatol P. Spork and Timothy J. Donohoe
Organic and Biomolecular Chemistry, 2015, 13, 4367-4373
DOI: 10.1039/C5OB00055F
Harish K. Potukuchi, Anatol P. Spork and Timothy J. Donohoe
Organic and Biomolecular Chemistry, 2015, 13, 4367-4373
DOI: 10.1039/C5OB00055F
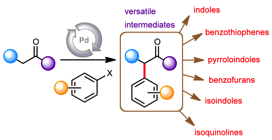
169 Hydrogen borrowing and interrupted hydrogen borrowing reactions of ketones and methanol, catalysed by iridium
Di Shen, Darren L. Poole, Camilla C. Shotton, Anne F. Kornahrens, Mark P. Healy and Timothy J. Donohoe,
Angewandte Chemie Int. Ed., 2015, 54, 1642-1645
DOI: 10.1002/anie.201410391
Di Shen, Darren L. Poole, Camilla C. Shotton, Anne F. Kornahrens, Mark P. Healy and Timothy J. Donohoe,
Angewandte Chemie Int. Ed., 2015, 54, 1642-1645
DOI: 10.1002/anie.201410391
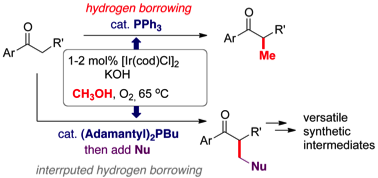
168 Short and efficient syntheses of protoberberine alkaloids using palladium catalyzed enolate arylation
Alice E. Gatland, Ben S. Pilgrim, Panayiotis A. Procopiou and Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2014, 53, 14555-14558.
DOI: 10.1002/anie.201409164
Alice E. Gatland, Ben S. Pilgrim, Panayiotis A. Procopiou and Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2014, 53, 14555-14558.
DOI: 10.1002/anie.201409164
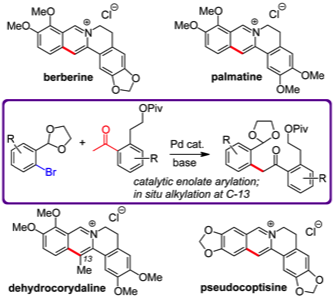
167 Love-Hate ligands for high resolution analysis of strain in ultra-stable protein:small molecule interaction
Michael Fairhead, Di Shen, Louis K. M. Chan, Ed D. Lowe, Timothy J. Donohoe, Mark Howarth
Bioorganic & Medicinal Chemistry, 2014, 22, 5476-5486
DOI: 10.1016/j.bmc.2014.07.029
Howarth Lab
Michael Fairhead, Di Shen, Louis K. M. Chan, Ed D. Lowe, Timothy J. Donohoe, Mark Howarth
Bioorganic & Medicinal Chemistry, 2014, 22, 5476-5486
DOI: 10.1016/j.bmc.2014.07.029
Howarth Lab
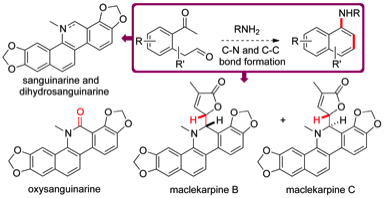
166 New methods for the synthesis of naphthyl amines; application to the synthesis of dihydrosanguinarine, sanguinarine, oxysanguinarine and (±)-maclekarpines B and C
Matthew R. Tatton, Iain Simpson and Timothy J. Donohoe
Chem. Commun., 2014, 50, 11314-11316
DOI: 10.1039/C4CC05209A
Matthew R. Tatton, Iain Simpson and Timothy J. Donohoe
Chem. Commun., 2014, 50, 11314-11316
DOI: 10.1039/C4CC05209A
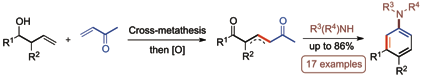
165 De novo Synthesis of Multi-Substituted Aryl Amines using Alkene Cross Metathesis
Matthew Tatton, Iain Simpson and Timothy J. Donohoe
Organic Letters, 2014, 16, 1920-1923.
DOI: 10.1021/ol500441q
Matthew Tatton, Iain Simpson and Timothy J. Donohoe
Organic Letters, 2014, 16, 1920-1923.
DOI: 10.1021/ol500441q
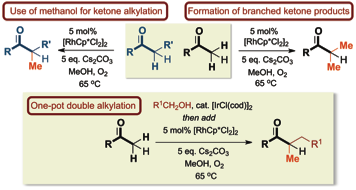
164 Rhodium-Catalyzed Ketone Methylation Using Methanol Under Mild Conditions: Formation of Alpha-Branched Products
Louis K. M. Chan, Darren L. Poole, Di Shen, Mark P. Healy and Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2014, 53, 761-765.
Designated as a HOT paper, featured as the back cover
DOI: 10.1002/anie.201307950
Louis K. M. Chan, Darren L. Poole, Di Shen, Mark P. Healy and Timothy J. Donohoe
Angewandte Chemie Int. Ed., 2014, 53, 761-765.
Designated as a HOT paper, featured as the back cover
DOI: 10.1002/anie.201307950
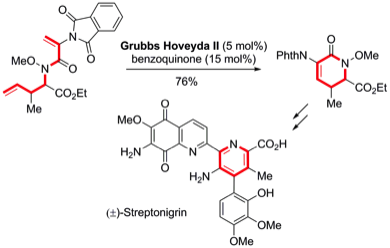
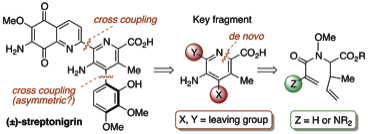
163 Total Synthesis of the Antitumor Antibiotic (+/-)-Streptonigrin: First and Second Generation Routes for de-novo Pyridine Formation using Ring Closing Metathesis
Timothy Donohoe, Christopher Jones, Anne Kornahrens, Luiz Barbosa, Louise Walport, Matthew Tatton, Michael O'Hagan, Akshat Rathi, David Baker
J. Org. Chem. 2013, 78, 12338-12350.
Selected as a Featured Article.
DOI: 10.1021/jo402388f
Timothy Donohoe, Christopher Jones, Anne Kornahrens, Luiz Barbosa, Louise Walport, Matthew Tatton, Michael O'Hagan, Akshat Rathi, David Baker
J. Org. Chem. 2013, 78, 12338-12350.
Selected as a Featured Article.
DOI: 10.1021/jo402388f
162 Modular isoquinoline synthesis using catalytic enolate arylation and in-situ functionalization
Ben Pilgrim, Alice Gatland, Charlie McTernan, Panayiotis Procopiou, Timothy Donohoe
Organic Letters, 2013, 15, 6190-6193.
DOI: 10.1021/ol4030309
Ben Pilgrim, Alice Gatland, Charlie McTernan, Panayiotis Procopiou, Timothy Donohoe
Organic Letters, 2013, 15, 6190-6193.
DOI: 10.1021/ol4030309
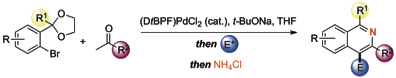
161 Tethered Aminohydroxylation: Synthesis of the β-Amino Acid of Microsclerodermins A and B
Robert D. C. Pullin , Akshat H. Rathi, Ekaterina Y. Melikhova, Christian Winter , Amber L. Thompson, and Timothy J. Donohoe
Organic Letters, 2013, 15, 5492-5495.
DOI: 10.1021/ol402638n
Robert D. C. Pullin , Akshat H. Rathi, Ekaterina Y. Melikhova, Christian Winter , Amber L. Thompson, and Timothy J. Donohoe
Organic Letters, 2013, 15, 5492-5495.
DOI: 10.1021/ol402638n
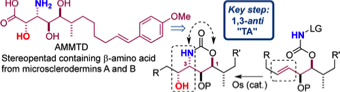
160 Lithium-4,4'-Di-tert-butylbiphenylide
Christopher R. Jones and Timothy J. Donohoe
Encyclopedia of Reagents for Organic Synthesis, 2013
Christopher R. Jones and Timothy J. Donohoe
Encyclopedia of Reagents for Organic Synthesis, 2013
159 Vinyl Weinreb Amides: A Versatile Alternative to Vinyl Ketone Substrates for Heck Arylation
David B. Baker, Peter T. Gallagher and Timothy J. Donohoe
Tetrahedron 2013, 69, 3690-3697.
DOI: 10.1016/j.tet.2013.03.009
David B. Baker, Peter T. Gallagher and Timothy J. Donohoe
Tetrahedron 2013, 69, 3690-3697.
DOI: 10.1016/j.tet.2013.03.009
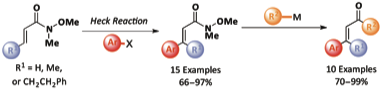
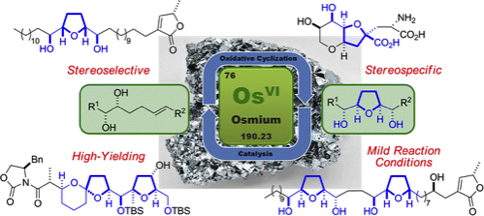
158 Osmium-Catalyzed Oxidative Cyclization of Dienes and Their Derivatives
Ben S. Pilgrim and Timothy J. Donohoe
J. Org. Chem. 2013, 78, 2149-2167.
Work from this paper featured in the cover picture for JOC.
DOI: 10.1021/jo302719y
Ben S. Pilgrim and Timothy J. Donohoe
J. Org. Chem. 2013, 78, 2149-2167.
Work from this paper featured in the cover picture for JOC.
DOI: 10.1021/jo302719y
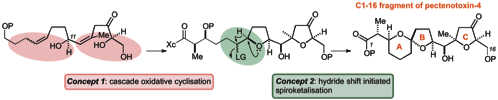
157 Interplay of cascade oxidative cyclisation and hydride shifts in the synthesis of the ABC spiroketal ring system of pectenotoxin-4
Timothy, J. Donohoe and Radek Lipinski
Angew. Chem. Int. Ed. 2013, 52, 2491-2494.
Designated as a Hot Article.
DOI: 10.1002/anie.201208919
Timothy, J. Donohoe and Radek Lipinski
Angew. Chem. Int. Ed. 2013, 52, 2491-2494.
Designated as a Hot Article.
DOI: 10.1002/anie.201208919
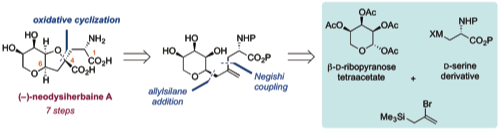
156 Oxidative cyclisation for the synthesis of complex THF-containing natural products
Timothy, J. Donohoe, Robert D. C. Pullin, Radoslaw M. Lipinski
Pure & Applied Chem. 2013, 85, 1175-1184.
DOI: 10.1351/PAC-CON-12-10-25
Timothy, J. Donohoe, Robert D. C. Pullin, Radoslaw M. Lipinski
Pure & Applied Chem. 2013, 85, 1175-1184.
DOI: 10.1351/PAC-CON-12-10-25
155 Partial and complete reduction of pyrroles, furans, thiophenes and their benzo-analogues and of aromatic heterocycles containing more than one heteroatom
Timothy J. Donohoe,* Christopher Jones and Christian Winter
Comprehensive Organic Synthesis II, Ed. G. Molander and P. Knochel, Elsevier, 2013, in the press.
Timothy J. Donohoe,* Christopher Jones and Christian Winter
Comprehensive Organic Synthesis II, Ed. G. Molander and P. Knochel, Elsevier, 2013, in the press.
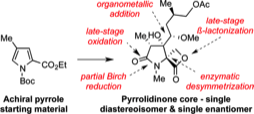
154 Asymmetric Synthesis of the Fully Elaborated Pyrrolidone Core of Oxazolomycin A
Timothy J. Donohoe, Timothy J. C. O'Riordan, Manuel Peifer, Christopher R. Jones, Timothy J. Miles
Organic Lett. 2012, 14, 5460-5463.
DOI: 10.1021/ol302541j
Timothy J. Donohoe, Timothy J. C. O'Riordan, Manuel Peifer, Christopher R. Jones, Timothy J. Miles
Organic Lett. 2012, 14, 5460-5463.
DOI: 10.1021/ol302541j
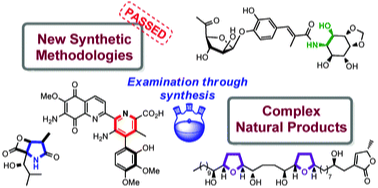
153 Natural Product Synthesis as a Challenging Test of Newly Developed Methodology
Timothy J. Donohoe, Robert D. C. Pullin
Chemical Commun. 2012, 48, 11924-11938
DOI: 10.1039/C2CC36040C
Timothy J. Donohoe, Robert D. C. Pullin
Chemical Commun. 2012, 48, 11924-11938
DOI: 10.1039/C2CC36040C
152 Synthesis of substituted isoquinolines utilizing palladium-catalyzed
α-arylation of ketones
Timothy J. Donohoe, Ben S. Pilgrim, Geraint R. Jones and José A. Bassuto
Proc. Natl. Acad. Sci. US, 2012, 109, 11605-11608.
DOI: 10.1073/pnas.1206532109
α-arylation of ketones
Timothy J. Donohoe, Ben S. Pilgrim, Geraint R. Jones and José A. Bassuto
Proc. Natl. Acad. Sci. US, 2012, 109, 11605-11608.
DOI: 10.1073/pnas.1206532109
151 A green approach to Fenton chemistry: Hydroxylation of salicylic acid in aqueous medium using electrogeneration of Fenton's Reagent
Janjira Panchompoo, Leigh Aldous, Mikhail Kabeshov, Ben S. Pilgrim, Timothy J. Donohoe and Richard G. Compton
New. J. Chem. 2012, 36, 1265-1272.
DOI: 10.1039/C2NJ21007J
150 Hydrogen-Bonding-Mediated Directed Osmium Dihydroxylation
Timothy J. Donohoe, Carole J. R. Bataille, Paolo Innocenti
Organic Reactions, 2012, 76, 1-47.
DOI: 10.1002/0471264180.or076.01
149 Olefin Cross-Metathesis for the Synthesis of Heteroaromatic Compounds
Timothy J. Donohoe, John F. Bower and Louis K. M. Chan
Org. Biomol. Chem. 2012, 10, 1322-1328.
Designated as a Hot Article.
DOI: 10.1039/C2OB06659A
148 Tethered Aminohydroxylation Reaction and its Application to Total Synthesis
Timothy J. Donohoe, Cedric K. A. Callens, Adam R. Lacy, Christian Winter
Eur. J. Org. Chem. 2012, 4, 655-663
(plus cover picture for this issue)
DOI: 10.1002/ejoc.201101464
147 Direct Preparation of thiazoles, imidazoles, imidazopyridines and thiazolidines from alkenes
Timothy J. Donohoe, Mikhail Kabeshov, Akshat Rathi and Ian E. D. Smith
Org. Biomol. Chem. 2012, 10, 1093-1101.
DOI: 10.1039/C1OB06587D
146 Tethered aminohydroxylation
Timothy J. Donohoe and Stefanie Mesch
Asymmetric Synthesis – The Essentials II. Edited by Mathias Christmann and Stefan Brase, WILEY-VCH, 2012, 17-26.
145 Amino Acid-Based Reoxidants for Aminohydroxylation: Application to the Construction of Amino Acid-Amino Alcohol Conjugates
Timothy J. Donohoe, Cedric K. A. Callens, Aida Flores, Stefanie Mesch, Darren L. Poole and Ishmael A. Roslan
Angew. Chem. Int. Ed. 2011, 50, 10957-10960.
DOI: 10.1002/anie.201103293
144 Synthesis of 2,4,6-Trisubstituted Pyridines via an Olefin Cross-Metathesis/Heck-Cyclisation-Elimination Sequence
Timothy J. Donohoe, John F. Bower, David B. Baker, José A. Basutto, Louis K. M. Chan and Peter Gallagher
Chemical Commun. 2011, 47, 10611-10613.
DOI: 10.1039/C1CC14257G
143 Total synthesis of (±)-streptonigrin: de novo construction of a pentasubstituted pyridine using ring-closing metathesis.
Timothy J. Donohoe, Christopher R. Jones, and Luiz C. A. Barbosa
J. Am. Chem. Soc. 2011, 133, 16418–16421.
DOI: 10.1021/ja207835w
142 Surface plasmon resonance imaging of glycoarrays identifies novel and unnatural carbohydrate-based ligands for potential ricin sensor development
Margherita Fais, Rositsa Karamanska, Sarah Allman, Shirley A. Fairhurst, Paolo Innocenti, Antony J. Fairbanks, Timothy J. Donohoe, Benjamin G. Davis, David A. Russell and Robert A. Field
Chemical Science, 2011, 2, 1952-1959.
DOI: 10.1039/C1SC00120E
141 The Influence of Exocyclic Stereochemistry on the Tethered Aminohydroxylation Reaction
Timothy J. Donohoe, Adam R. Lacy, Akshat H. Rathi, and Daryl S. Walter
Chemistry: An Asian Journal, 2011, 6, 3214-3222.
DOI: 10.1002/asia.201100497
140 A short and efficient synthesis of neodysiherbaine A using catalytic oxidative cyclisation
Timothy J. Donohoe, Paul Winship, Matthew R. Tatton and Peter Szeto
Angew. Chem. Int. Ed. 2011, 50, 7604-7606.
DOI: 10.1002/anie.201102525
139 Dehydrogenation of Cyclic-Thioethers Bound to a [Rh(diphosphine)]+ Fragment
Romeao Dallanegra, Ben S. Pilgrim, Adrian B. Chaplin, Timothy J. Donohoe and Andrew S. Weller
Dalton Transactions, 2011, 40, 6626-6628.
DOI: 10.1039/C1DT10503E
138 Exerting Control over the Acyloin Reaction
Timothy J. Donohoe, Ali Jahanshahi, Michael J. Tucker, Farrah L. Bhatti, Ishmael A. Roslan, Mikhail Kabeshov and Gail Wrigley
Chemical Commun. 2011, 47, 5849-5851.
DOI: 10.1039/C1CC11654A
137 Intramolecular Hydride Addition to Pyridinium Salts: New Routes to Enantiopure Dihydropyridones
Timothy J. Donohoe, Matthew J. Connolly, Akshat H. Rathi and Lesley Walton
Organic Lett. 2011, 13, 2074-2077
DOI: 10.1021/ol200478p
136 Palladium nanoparticle-modified carbon nanotubes for electrochemical hydrogenolysis in ionic liquids
Yao Meng, Leigh Aldous, Ben S. Pilgrim, Timothy J. Donohoe and Richard G. Compton
New Journal of Chemistry, 2011, 35, 1369-1375.
DOI: 10.1039/C1NJ20070D
135 Heteroaromatic Synthesis via Olefin Cross-Metathesis: Entry to Polysubstituted Pyridines
Timothy J. Donohoe, José Basutto, Akshat H. Rathi and John F. Bower
Organic Lett. 2011, 13, 1036-1039.
DOI: 10.1021/ol103088r
134 Recent Developments in Methodology for the Direct Oxyamination of Olefins
Timothy J. Donohoe, Aida Flores, Cedric K.A. Callens, Adam J. Lacy, Akshat H. Rathi
Chem. Eur. J., 2011, 17, 58-76.
DOI: 10.1002/chem.201002323
133 Osmium-free direct syn-dihydroxylation of alkenes
Carole J. R. Bataille and Timothy J. Donohoe
Chem. Soc. Rev. 2011, 40, 114-128.
DOI: 10.1039/B923880H
132 Direct preparation of heteroaromatic compounds from alkenes
Timothy J. Donohoe, Mikhail A. Kabeshov, Akshat H. Rathi and Ian E.D. Smith
Synlett, 2010, 19, 2956-2958.
DOI: 10.1055/s-0030-1259034
131 Identification of Epigenetic DNA Modifications with a Protein Nanopore
Emma V. B. Wallace, David Stoddart, Andrew J. Heron, Ellina Mikhailova, Giovanni Maglia, Timothy J. Donohoe, Hagan Bayley
Chemical Commun. 2010, 46, 8195-8197.
DOI: 10.1039/C0CC02864A
130 A Novel Oxidative Cyclisation onto Vinyl Silanes
Timothy J. Donohoe, Paul C. M. Winship, Ben S. Pilgrim, Daryl S. Walter, Cedric K. A. Callens
Chemical Commun. 2010, 46, 7310-7312.
DOI: 10.1039/c0cc01342k
129 Olefin cross-metathesis based approaches to furans: procedures for the preparation of di- and trisubstituted variants
Timothy J. Donohoe, John F. Bower and José A. Basutto
Nature: Protocols, 2010, 5, 2005-2010.
DOI: 10.1038/nprot.2010.147
128 Substituted Pyrroles via Olefin Cross-Metathesis
Timothy J. Donohoe, Nicholas J. Race, John F. Bower and Cedric K. A. Callens
Organic Lett. 2010, 12, 4094-4097.
DOI: 10.1021/ol101681r
127 Synthesis of cylindricine C and a formal synthesis of cylindricine A
Timothy J. Donohoe, Ptoton M. Brian, Gráinne C. Hargaden and Timothy J.C. O'Riordan
Tetrahedron (Steven Ley Prize Issue), 2010, 66, 6411-6420.
DOI: 10.1016/j.tet.2010.05.044
126 An expedient route to substituted furans via olefin cross metathesis
Timothy J. Donohoe and John F. Bower
Proc. Natl. Acad. Sci. US, 2010, 107, 3373-3376.
This work has been highlighted in PNAS, Science and Nature Chemistry journals with a commentary.
DOI: 10.1073/pnas.0913466107
125 New modes for the osmium-catalyzed oxidative cyclization
Timothy. J. Donohoe, Cedric K.A. Callens, Jeremy Parker and Peter J. Lindsay-Scott
Organic Lett. 2010, 12, 1060-1063.
This paper was recently highlighted in the literature, see: Synfacts, 2010, 6, 683.
DOI: 10.1021/ol100046a
124 Regioselective nucleophilic addition to pyridinium salts: a new route to substituted dihydropyridones
Timothy J. Donohoe, Matthew J. Connolly and Lesley Walton
Organic Lett. 2009, 11, 5562-5565.
DOI: 10.1021/ol902402v
123 Author profile
Timothy J. Donohoe
Angew. Chem. Int. Ed. 2009, 48, 7119.
DOI: 10.1002/anie.200903879
122 Ring-closing metathesis for the synthesis of heteroaromatics: evaluating routes to pyridines and pyridazines
Timothy J. Donohoe, John F. Bower, José A. Basutto, Lisa P. Fishlock, Panayiotis A. Procopiou and Cedric K.A. Callens
Tetrahedron, 2009, 8969–8980.
DOI: 10.1016/j.tet.2009.07.076
121 Concise syntheses of the natural products (+)-sylvaticin and (+)-cis-sylvaticin
Timothy J. Donohoe, Robert M. Harris, Oliver Williams, Grainne C. Hargaden, Jeremy Burrows, Jeremy Parker
J. Am. Chem. Soc. 2009, 131, 12854-12861.
DOI: 10.1021/ja9049959
120 Synthesis of (–)-Hygromycin A: Application of Mitsunobu Glycosylation and Tethered Aminohydroxylation
Timothy J. Donohoe, Aida Flores, Carole J. Bataille and Fátima Churruca
Angew. Chem. Int. Ed. 2009, 48, 6507-6510.
DOI: 10.1002/ange.200902840
119 A Lewis Acid Promoted Oxidative Cyclisation
Timothy J. Donohoe, Paul C. M. Winship and Daryl S. Walter
J. Org. Chem. 2009, 74, 6394-6397.
DOI: 10.1021/jo901243y
118 Osmium-mediated Oxidative Cyclisations: a study into the range of initiators that facilitate cyclisation
Timothy J. Donohoe, Katherine M. P. Wheelhouse (née Gosby), Peter J. Lindsay-Scott, Gwydion H. Churchill, Matthew J. Connolly, Sam Butterworth and Paul A. Glossop
Chemistry: An Asian Journal, 2009, 4, 1237-1247.
DOI: 10.1002/asia.200900168
117 Electrochemistry in tetrahydrofuran and at low temperature: protocol, procedures and methods
Ronan Baron, Neil M. Kershaw, Timothy J. Donohoe and Richard G. Compton
Journal of Phys. Org. Chem. 2009, 22, 1136-1141.
DOI: 10.1002/poc.1574
116 The tethered aminohydroxylation (TA) reaction of amides
Timothy J. Donohoe, Cedric K.A. Callens and Amber L. Thompson
Organic Lett. 2009, 11, 2305-2307.
DOI: 10.1021/ol900631y
115 The effect of ortho-substitution on the efficacy of biphenyls in mediating electron transfer from lithium
Timothy J. Donohoe, Neil M. Kershaw, Ronan Baron and Richard G. Compton
Tetrahedron, 2009, 65, 5377-5384.
DOI: 10.1016/j.tet.2009.04.057
114 Synthesis of substituted pyridines and pyridazines via ring closing metathesis
Timothy J. Donohoe, Lisa P. Fishlock, José A. Basutto, John F. Bower, Panayiotis A. Procopiou and Amber L. Thompson
Chemical Commun. 2009, 21, 3008-3010.
DOI: 10.1039/b904363b
113 Tandem catalysis in the polycyclisation of dienes to produce multi-substituted tetrahydrofurans
Timothy J. Donohoe, Peter J. Lindsay-Scott and Jeremy S. Parker
Tetrahedron Letters, 2009, 50, 3523-3526
DOI: 10.1016/j.tetlet.2009.03.027
112 Ruthenium catalysed isomerization of terminal Olefins: applications to synthesis
Timothy J. Donohoe, Timothy J.C. O'Riordan and Carla P. Rosa
Angew. Chem. Int. Ed. 2009, 48, 1014-1017.
DOI: 10.1002/anie.200804617
111 Quantitative voltammetry of the reduction of methyl benzoate in THF reveals strong ion pairing of the radical anion with tetra-n-butyl cations
Ronan Baron, Neil M. Kershaw, Timothy J. Donohoe and Richard G. Compton
Journal of Phys. Org. Chem. 2009, 22, 247-253.
DOI: 10.1002/poc.1462
110 Synthesis of (+)-DGDP and (–)-7-Epialexine
Timothy J. Donohoe, Matthew D. Cheeseman, Timothy J.C. O'Riordan and Jessica A. Kershaw
Org. Biomol. Chem. 2008, 6, 3896-3898.
DOI: 10.1039/b815332a
109 Alkali metal reductions of organic molecules: why mediated electron transfer from Lithium is faster than direct reduction
Neil Rees, Ronan Baron, Neil Kershaw, Timothy J. Donohoe, Richard J. Compton
J. Am. Chem. Soc. 2008, 130, 12256-12257.
DOI: 10.1021/ja805086w
108 Flexible strategy for the synthesis of pyrrolizidine alkaloids
Timothy J. Donohoe, Rhian Thomas, Matthew Cheeseman, Caroline Rigby, Ian Linney and Gurdip Bhalay
Organic Lett. 2008, 10, 3615-3618.
DOI: 10.1021/ol801415d
107 Synthesis of (–)-(Z)-deoxypukalide
Timothy J. Donohoe, Alan Ironmonger and Neil M. Kershaw
Angew. Chem. Int. Ed. 2008, 47, 7314-7316.
DOI: 10.1002/anie.200802703
106 Ring-Closing Metathesis as a Key Step in the Synthesis of 2-Pyridones and Pyridine Triflates
Timothy J. Donohoe, Lisa P. Fishlock and Panayiotis A. Procopiou
Synthesis, 2008, 129, 2665-2667 (Practical Synthetic Procedure)
DOI: 10.1055/s-2008-1067180
105 Ring-closing metathesis: novel routes to aromatic heterocycles
Timothy J. Donohoe, Lisa P. Fishlock and Pan A. Procopiou
Chemistry: A European Journal, 2008, 14, 5716-5726
DOI: 10.1002/chem.200800130
104 Pyridine-N-Oxide as a mild reoxidant which transforms Osmium catalysed oxidative cyclisation
Timothy J. Donohoe, Katherine M.P. Wheelhouse (née) Gosby, Peter J. Lindsay-Scott, Paul A. Glossop, Ian A. Nash and Jeremy S. Parker
Angew. Chem. Int. Ed. 2008, 47, 2872-2875.
DOI: 10.1002/anie.200705425
103 Hydride shift generated oxonium ions: evidence for mechanism and intramolecular trapping experiments to form trans THF derivatives
Timothy J. Donohoe, Oliver Williams and Gwydion H. Churchill
Angew. Chem. Int. Ed. 2008, 47, 2869-2871.
DOI: 10.1002/anie.200705340
102 A metathesis-based approach to the synthesis of 2-Pyridones and Pyridines
Timothy J. Donohoe, Lisa P. Fishlock and Pan A. Procopiou
Organic Lett. 2008, 10, 285-288.
DOI: 10.1021/ol702684d
101 Flexible metathesis-based approaches to highly functionalised furans and pyrroles
Timothy J. Donohoe, Neil M. Kershaw, Allan J. Orr, Katherine M. P. Wheelhouse (née Gosby), Lisa P. Fishlock, Adam R. Lacy, Matilda Bingham and Panayiotis A. Procopiou
Tetrahedron, 2008, 64, 809-820.
DOI: 10.1016/j.tet.2007.09.087
100 Electrosynthetic reduction of 1-iodoadamantane forming 1,1'-biadamantane and adamantane in aprotic solvents: Insonation switches the mechanism from dimerisation to exclusive monomer formation
Christopher A. Paddon, Farrah L. Bhatti, Timothy J. Donohoe and Richard G. Compton
Ultrasonics Sonochemistry, 2007, 14, 502-508.
DOI: 10.1016/j.ultsonch.2006.11.007
99 Cryoelectrochemical reduction of a phenyl sulfide in tetrahydrofuran: mediated reduction gives different products compared to direct reduction
Alexander V. Burasov, Christopher A. Paddon, Farrah L. Bhatti, Timothy J. Donohoe and Richard G. Compton
J. Phys. Org. Chem. 2007, 20, 144-150.
DOI: 10.1002/poc.1138
98 Electrocatalytic reduction of alkyl iodides in tetrahydrofuran at silver electrodes
Christopher A. Paddon, Farrah L. Bhatti, Timothy J. Donohoe and Richard G. Compton
J. Phys. Org. Chem. 2007, 20, 115-121.
DOI: 10.1002/poc.1133
97 Kinetics and thermodynamics of the Li/Li+ couple in tetrahydrofuran at low temperatures (195-295 K)
Christoper A. Paddon, Sarah E. Ward Jones, Farrah L. Bhatti, Timothy J. Donohoe and Richard G. Compton
J. Phys. Org. Chem. 2007, 20, 677-684.
DOI: 10.1002/poc.1230
96 A concise and efficient synthesis of (-)-Allosamizoline
Timothy J. Donohoe and Carla P. Rosa
Organic Lett. 2007, 9, 5509-5511.
DOI: 10.1021/ol702449m
95 Mediated electron transfer from lithium investigated voltammetrically in tetrahydrofuran: why are some mediators more effective reducing reagents than others?
Natasha C.L. Wood, Christopher A. Paddon, Farrah L. Bhatti, Timothy J. Donohoe and Richard G. Compton
J. Phys. Org. Chem. 2007, 20, 732-742.
DOI: 10.1002/poc.1232
94 The partial reduction of electron-deficient pyrroles: procedures describing both Birch (Li/NH3) and ammonia-free (Li/DBB) conditions
Timothy J. Donohoe, Rhian E. Thomas
Nature: Protocols, 2007, 2, 1888-1895.
Cover picture for this issue
DOI: 10.1038/nprot.2007.245
93 The Tethered Aminohydroxylation: Dramatic Improvements to the Process
Timothy J. Donohoe, Carole J. R. Bataille, William Gattrell, Johannes Kloesges and Emilie Rossignol
Organic Lett. 2007, 9, 1725-1728
DOI: 10.1021/ol070430v
92 A Metathesis Based Approach to the Synthesis of Furans
Timothy J. Donohoe, Lisa P. Fishlock, Adam R. Lacy, and Panayiotis A. Procopiou
Organic Lett. 2007, 9, 953-956
DOI: 10.1021/ol062933r
91 Coulometry on the Voltammetric Timescale: Microdisk Potential-Step Chronoamperometry in Aprotic Solvents Reliably Measures the Number of Electrons Transferred in an Electrode Process Simultaneously with the Diffusion Coefficients of the Electroactive species
Christopher A. Paddon, Debbie S. Silvester, Farrah L. Bhatti, Timothy J. Donohoe, Richard G. Compton
Electroanalysis, 2007, 19, 11-22.
DOI: 10.1002/elan.200603667
90 Partial Reduction of Pyrroles: Application to Natural Product Synthesis
Timothy J. Donohoe, and Rhian E. Thomas
The Chemical Record, 2007, 7, 180-190.
DOI: 10.1002/tcr.20115
89 Synthesis of the Pyrrolidinone Core of KSM-2690 B
Timothy J. Donohoe, Jessica Chiu, Rhian E Thomas
Organic Lett. 2007, 9, 421-424.
DOI: 10.1021/ol062705x
88 Total Synthesis of (+)-cis-Sylvaticin: Double Oxidative Cyclisation Reactions Catalyzed by Osmium
Timothy J. Donohoe, Robert M. Harris, Jeremy Burrows and Jeremy Parker
J. Am. Chem. Soc. 2006, 128, 13704-13705.
DOI: 10.1021/ja0660148
87 Stereoselective Synthesis of Pyrrolidines: Catalytic Oxidative Cyclisations Mediated by Osmium
Timothy J. Donohoe, Gwydion H. Churchill, Katherine M. P. Wheelhouse (née Gosby) and Paul A. Glossop
Angew. Chem. Int. Ed. 2006, 45, 8025-8028.
DOI: 10.1002/anie.200603240
86 Cryo-electrochemistry in tetrahydrofuran: The regioselective electrochemical reduction of a phenyl sulfone: Fast-scan cyclic voltammetry investigations
Nicole Fietkaua, Christopher A. Paddon, Farrah L. Bhattib, Timothy J. Donohoe and Richard G. Compton
Journal of Electroanalytical Chemistry 2006, 593, 131-141.
DOI: 10.1016/j.jelechem.2006.03.004
85 Cryovoltammetrically probing functional group reductive cleavage: Alkyl-sulfur versus aryl-sulfur bond cleavage in an alkyl naphthyl thioether under single electron transfer is temperature switchable
Christopher A. Paddon, Farrah L. Bhatti, Timothy J. Donohoe and Richard G. Compton
Chem. Commun. 2006, 3402-2404.
DOI: 10.1039/B606638K
84 Highlights of Natural Product Synthesis
Timothy J. Donohoe, Carole J. R. Bataille and Gwydion H. Churchill
Annual Rep. Prog. Chem. Sect. B, 2006, 102, 98-122.
DOI: 10.1039/B515095G
83 An Enzymatic Approach to the Desymmetrisation of Disubstituted Pyrrolines
Timothy J. Donohoe, Caroline L. Rigby, Rhian E. Thomas, William F. Nieuwenhuys, Farrah L. Bhatti, Andrew R. Cowley, Gurdip Bhalay and Ian D. Linney
J. Org. Chem. 2006, 71, 6298-6301.
DOI: 10.1021/jo060926a
82 Cryo-electrochemistry in tetrahydrofuran: The electrochemical reduction of a phenyl thioether: [(3-{[ trans -4-(Methoxymethoxy)cyclohexyl]oxy}propyl)thio]benzene
Christopher A. Paddon, Farrah L. Bhatti, Timothy J. Donohoe and Richard G. Compton
Journal of Electroanalytical Chemistry, 2006, 589, 187-194.
DOI: 10.1016/j.jelechem.2006.02.010
81 New Osmium-Based Reagent for the Dihydroxylation of Alkenes
Timothy J. Donohoe, Robert M. Harris, Sam Butterworth, Jeremy N. Burrows, Andrew Cowley, and Jeremy S. Parker
J. Org. Chem. 2006, 71, 4481-4489.
DOI: 10.1021/jo060301c
80 N-Sulfonyloxy Carbamates as Re-oxidants for the Tethered Aminohydroxylation Reaction
Timothy J. Donohoe, Majid J. Chughtai, David J. Klauber, David Griffin and Andrew D. Campbell
J. Am. Chem. Soc. 2006, 128, 2514-2515.
DOI: 10.1021/ja057389g
79 The Ammonia-Free Partial Reduction of Substituted Pyridinium Salts
Timothy J. Donohoe, Dale J. Johnson, Laura H. Mace, Rhian E. Thomas, Jessica Y. K. Chiu, Jason S. Rodrigues, Richard G. Compton, Craig E. Banks, Peter Tomcik, Mark J. Bamford and Osamu Ichihara
Org. Biomol. Chem. 2006, 4, 1071-1084.
DOI: 10.1039/B517462G
78 Ring Closing Metathesis as a Basis for the Construction of Aromatic Compounds
Timothy J. Donohoe,* Allan J. Orr and Matilda Bingham
Angew. Chem. Int Ed. 2006, 45, 2664-2670.
DOI: 10.1002/anie.200503512
77 A non-carbohydrate based approach to polyhydroxylated pyrrolidizines; total syntheses of the natural products hyacinthacine A1 and 1-epiaustraline
Timothy J. Donohoe, Herman O. Sintim and Jackie Hollinshead
J. Org. Chem. 2005, 70, 7297-7304.
DOI: 10.1021/jo050977s
76 Oxidative cyclisations of diols derived from 1,5-dienes: formation of enantiopure cis-tetrahydrofurans using catalytic osmium tetroxide and a formal synthesis of (+)-cis-solamin
Timothy J. Donohoe and Sam Butterworth
Angew. Chem. Int. Ed. 2005, 44, 4766-4768.
DOI: 10.1002/anie.200500513
75 A Metathesis Approach to Aromatic Heterocycles
Timothy J. Donohoe, Allan J. Orr, Katherine Gosby and Matilda Bingham
Eur. J. Org. Chem. 2005, 1969-1971.
DOI: 10.1002/ejoc.200500113
74 A Concise and Enantioselective Synthesis of the Aminocyclitol Core of Hygromycin A
Timothy J. Donohoe, Peter D. Johnson, Richard J. Pye and Martine Keenan
Organic Lett. 2005, 7, 1275-1277.
DOI: 10.1021/ol0473750
73 Utility of the ammonia-free Birch reduction of electron-deficient pyrroles: total synthesis of the 20S proteasome inhibitor, clasto-lactacystin beta-lactone
Timothy J. Donohoe, Herman O. Sintim, Leena Sisangia, Karl W. Ace, Paul M. Guyo, Andrew Cowley and John D. Harling
Chemistry: A European Journal, 2005, 11, 4227-4238.
DOI: 10.1002/chem.200401119
72 Partial Reduction of Pyridinium Salts as a Versatile Route to Dihydropyridones
Timothy J. Donohoe, D. Johnson, L. H. Mace, O. Ichihara and M. Bamford
Organic Lett. 2005, 7, 453-437.
DOI: 10.1021/ol0476624
71 Cryoelectrochemistry: Electrochemical reduction of 2(RS)-Methyl 1-(tert-butoxycarbonyl)-2-iodomethyl-2,5-dihydropyrrole-2-carboxylate
Craig E. Banks, Russell G. Evans, Jason Rodrigues, Peter G. Turner, Timothy J. Donohoe and Richard G. Compton
Tetrahedron, 2005, 61, 2365-2372.
DOI: 10.1016/j.tet.2005.01.022
70 Enantioselective Partial Reduction of 2,5-Disubstituted Pyrroles via a Chiral Protonation Approach
Timothy J. Donohoe, Catherine E. Headley, Caroline L. Rigby, Graeme C. Freestone, Rick P. C. Cousins and Gurdip Bhlay
Organic Lett. 2004, 6, 3055-3058.
DOI: 10.1021/ol049014q
69 Efficient Acyclic Stereocontrol Using the Tethered Aminohydroxylation Reaction
Timothy J. Donohoe, Peter D. Johnson, Martine Keenan and Richard J. Pye
Organic Lett. 2004, 6, 2583-2585.
DOI: 10.1021/ol049136i
68 An Efficient Synthesis of (±)-1 Epiaustraline
Timothy J. Donohoe and Herman O. Sintim
Organic Lett. 2004, 6, 2003-2006.
DOI: 10.1021/ol049397s
67 Rearrangement of Pyrrolines Derived from the Birch Reduction of Electron-Deficient Pyrroles: Radical Ring-Expansion to Substituted Tetrahydropyridines
Timothy J. Donohoe, Rick P. C. Cousins and Peter Turner
Chem. Commun. 2004, 1422-1423.
DOI: 10.1039/B404002C
66 Low Temperature Electrochemistry as a Mechanistic Probe for the Partial Reduction of Heterocycles
Timothy J. Donohoe, Richard Compton, Dale Johnson and Jay D. Wadhawan
Tetrahedron, 2004, 60, 5945-5952.
DOI: 10.1016/j.tet.2004.05.027
65 An Efficient Synthesis of Lactacysin ß-Lactone
Timothy J. Donohoe, Leena Sisangia, Herman O. Sintim and John D. Harling
Angew. Chem. Int. Ed. 2004, 43, 2293-2296.
DOI: 10.1002/anie.200453843
64 Enantiopure Oxazolidinones as Chiral Acids in the Asymmetric Protonation of N-Boc Pyrrole Derived Enolates
Timothy J. Donohoe and David Carbery
Chem. Commun. 2004, 722-723.
DOI: 10.1039/B316719D
63 Synthesis of (±)-Secosyrin 1 and a Formal Synthesis of (–)-Secosyrin 1
Timothy J. Donohoe, John W. Fisher and Paul J. Edwards
Organic Lett. 2004, 6, 465-467.
DOI: 10.1021/ol0362313
62 Trichloro-oxazolines as activated donors for aminosugar coupling
Timothy J. Donohoe, James G. Logan and David D. P. Laffan
Organic Lett. 2003, 5, 4995 - 4998.
DOI: 10.1021/ol0359620
61 Calcium mobilisation and CCK secretion induced by modified fatty acids and latex microspheres reveal dual receptor mechanisms for lipid stimulation of STC-1 cells.
S. Kazmi, R. S. P. Benson, T. J. Donohoe, M. N. Jones, M. Wickham, D. G. Thompson, R. M. Case
Journal of Physiology, 2003, 553, 759-773.
DOI: 10.1113/jphysiol.2003.051680
60 Osmium Tetroxide
Timothy J. Donohoe, Robert Harris and Majid Chugtai
Encyclopedia of Reagents for Organic Synthesis, 2003.
DOI: 10.1002/047084289X.ro007.pub2
59 Scope of the Reductive Aldol Reaction: Application to Aromatic Carbocycles and Heterocycles
Timothy J. Donohoe, David House and K. W. Ace
Org. Biomol. Chem. 2003, 3749-3757.
DOI: 10.1039/B306937K
58 The Tethered Aminohydroxylation (TA) Reaction
Timothy J. Donohoe, Peter D. Johnson and Richard J. Pye
Org. Biomol. Chem. 2003, 1, 2025-2028.
DOI: 10.1039/b305189g
57 Scope of the Directed Dihydroxylation: Application to Cyclic Homoallylic Alcohols and Trihaloacetamides
Timothy J. Donohoe, Lee Mitchell, Michael J. Waring, Madeleine Helliwell, Andrew Bell, and Nicholas J. Newcombe
Org. Biomol. Chem. 2003, 1, 2173-2186.
DOI: 10.1039/b303081d
56 Flexibility in the Partial Reduction of 2,5-Disubstituted Pyrroles: Application to the Synthesis of DMDP
Timothy J. Donohoe, Catherine E. Headley, Rick P. C. Cousins and Andrew Cowley
Organic Lett. 2003, 5, 999-1002.
DOI: 10.1021/ol027504h
55 A General Oxidative Cyclisation of 1,5-Dienes Using Catalytic Osmium Tetroxide
Timothy J. Donohoe and Sam Butterworth
Angew. Chem. Int. Ed. 2003, 42, 948-951.
DOI: 10.1002/anie.200390253
54 Diastereoselective reductive aldol reactions of BOC-protected electron deficient pyrroles
Timothy J. Donohoe and David House
Tetrahedron Letters, 2003, 44, 1095-1098.
DOI: 10.1016/S0040-4039(02)02672-2
53 The Directed Dihydroxylation of Cyclic Allylic Alcohols and Trichloroacetamides Using OsO4/TMEDA
Timothy J. Donohoe, Kevin Blades, Peter R. Moore, Michael J. Waring, Jon J. G. Winter, Madeleine Helliwell, Nicholas J. Newcombe and Geoffrey Stemp
J. Org. Chem. 2002, 67, 7946-7956.
DOI: 10.1021/jo026161y
52 The Tethered Aminohydroxylation (TA) of Cyclic Allylic Carbamates
Timothy J. Donohoe, Peter D. Johnson, Andrew Cowley and Martine Keenan
J. Am. Chem. Soc. 2002, 124, 12934-12935.
DOI: 10.1021/ja0276117
51 Synthesis of enantiopure dihydropyranones: aldol-based ring expansion of dihydrofurans
Timothy J. Donohoe, Ali Raoof, Graeme C. Freestone, Ian D. Linney, Andrew Cowley and Madeleine Helliwell
Organic Lett. 2002, 4, 3059-3062.
DOI: 10.1021/ol0263595
50 Partial reduction of 3-heteroatom substituted 2-furoic acids: the role of an ortho group on viability and stereoselectivity
Timothy J. Donohoe, Andrew A. Calabrese, Jean-Baptiste Guillermin, Christopher. S. Frampton and Daryl Walter
J. Chem. Soc. Perkin Transactions 1, 2002, 1748-1756.
DOI: 10.1039/B203437A
49 Ammonia Free Reduction of Aromatic Compounds Using Lithium Di-tert-butylbiphenyl (LiDBB)
Timothy J. Donohoe and David House
J. Org. Chem. 2002, 67, 5015-5018.
DOI: 10.1021/jo0257593
48 Preparation of (+)-Nemorensic Acid and Approaches to Nemorensine using the Partial Reduction of Electron Deficient Furans
Timothy J. Donohoe, Jean-Baptiste Guillermin and Daryl S. Walter
J. Chem. Soc. Perkin Transactions 1, 2002, 1369-1375.
DOI: 10.1039/B202514K
47 Development of the Directed Dihydroxylation Reaction
Timothy J. Donohoe
Synlett, 2002, 8, 1223-1232.
DOI: 10.1055/s-2002-32947
46 Transformations of 3,4-disubstituted pyridines under dissolving metal conditions-partial reduction followed by radical cyclisation
Timothy J Donohoe, Laura Mace, Madeleine Helliwell and Osamu Ichihara
Synlett, 2002, 331-333.
DOI: 10.1055/s-2002-19757
45 Homoallylic alcohols and trichloroacetamides as hydrogen bond donors for directed dihydroxylation
Timothy J. Donohoe, L. Mitchell, M. J. Waring, M. Helliwell, A. Bell and N. J. Newcombe
Tetrahedron Letters, 2001, 42, 8951-8954.
DOI: 10.1016/S0040-4039(01)01999-2
44 The Regioselective Aminohydroxylation of Allylic Carbamates
Timothy J. Donohoe, Peter D. Johnson, Madeleine Helliwell, and Martine Keenan
Chem. Commun. 2001, 2078.
DOI: 10.1039/B107253F
43 Silyl substituted furans in the stereoselective Birch reduction
Timothy J Donohoe, Andrew A. Calabrese, Jean-Baptiste Guillermin and Daryl S. Walter
Tetrahedron Letters, 2001, 42, 5841.
DOI: 10.1016/S0040-4039(01)01151-0
42 Use of Dissolving Metals in the Partial Reduction of Pyridines: Formation of 2-Alkyl-1,2-Dihydropyridines
T. J. Donohoe, Andrew J. McRiner, Madeleine Helliwell and Peter Sheldrake
J. Chem. Soc. Perkin Transactions 1, 2001, 1435-1445.
DOI: 10.1039/B101662H
41 Partial Reduction of Annulated Heterocycles as a General Route to Medium Rings Containing Oxygen and Nitrogen
Timothy J. Donohoe, Ali Raoof, Ian D. Linney and Madeleine Helliwell
Organic Lett. 2001, 3, 861-864.
DOI: 10.1021/ol007035o
40 Hydrogen bonding control in the oxidative cyclisation of 1,5-dienes
Timothy J. Donohoe, Jonathan J. G. Winter, M. Helliwell and Geoffrey Stemp
Tetrahedron Letters, 2001, 42, 971-974.
DOI: 10.1016/S0040-4039(00)02225-5
39 Partial Reduction of Electron-Deficient Pyridines
T. J. Donohoe, A. J. McRiner and P. Sheldrake
Organic Lett. 2000, 2, 3861-3863.
DOI: 10.1021/ol0065930
38 Stereoselective Reduction of Chiral 2-Furoic Acid Derivatives Using Group I Metals in Ammonia
Timothy J. Donohoe, Andrew A. Calabrese, Clare A. Stevenson and Tamara Ladduwahetty
J. Chem. Soc. Perkin Transactions 1, 2000, 3724.
DOI: 10.1039/B006390H
37 Oxidation and Reduction in Organic Synthesis: OUP Chemistry Primer No. 6
Timothy J. Donohoe
Oxford University Press, 2000, ISBN 0198556640.
36 A Syn Selective Dihydroxylation of Cyclic Allylic Trichloroacetamides Using Catalytic Osmium Tetroxide
Kevin Blades, Timothy J. Donohoe, Jonathan J. G. Winter and Geoffrey Stemp
Tetrahedron Letters, 2000, 41, 4701-4704.
DOI: 10.1016/S0040-4039(00)00714-0
35 The Synthesis of (+)-Nemorensic Acid
Timothy J. Donohoe, J. -B. Guillermin, C. Frampton and D. S. Walters
J. Chem Soc., Chem. Commun. 2000, 465-466.
DOI: 10.1039/B000565G
34 The partial reduction of heterocycles: an alternative to the Birch reduction
Timothy J. Donohoe, Rakesh R. Harji and Rick P. C. Cousins
Tetrahedron Letters, 2000, 41, 1331-1334.
DOI: 10.1016/S0040-4039(99)02315-1
33 Stereoselective Reductive Alkylation of 2,5-disubstituted Pyrroles: A Role for Naphthalene in the Partial Reduction of Heterocycles
Timothy J. Donohoe, Rakesh R. Harji and Rick P. C. Cousins
Tetrahedron Letters, 2000, 41, 1327-1330.
DOI: 10.1016/S0040-4039(99)02314-X
32 Reductive Aldol Reactions on Aromatic Heterocycles
Timothy J. Donohoe, Karl W. Ace, Paul M. Guyo, Madeleine Helliwell and Jeffrey McKenna
Tetrahedron Letters, 2000, 41, 989-993.
DOI: 10.1016/S0040-4039(99)02224-8
31 Enhanced Stereoselectivity in the Catalytic Dihydroxylation of Acyclic Allylic Alcohols
Timothy J. Donohoe, Michael J. Waring, and Nicholas J. Newcombe
Synlett, 2000, 149-151.
DOI: 10.1055/s-2000-6457
30 1H- and 2H-Isoindoles
Timothy J. Donohoe
Science of Synthesis, Georg Thieme Verlag, 2000, Volume 10, 653-692. (Review)
29 Birch Reduction of Aromatic Heterocycles
Timothy J. Donohoe, P. M. Guyo and A. Raoof
Targets in Heterocyclic Systems, Italian Society of Chemistry, 1999, 3, 117-145 (Review).
28 Syn Stereocontrol in the Directed Dihydroxylation of Ayclic Allylic Alcohols
Timothy J. Donohoe, Michael J. Waring and Nicholas J. Newcombe
Tetrahedron Letters, 1999, 40, 6881-6884.
DOI: 10.1016/S0040-4039(99)01371-4
27 The Synthesis of (–)-cis and (–)-trans-Crobarbatic Acid
Timothy J. Donohoe, C. A. Stevenson M. Helliwell, R. Irshad and T. Ladduwahetty
Tetrahedron: Asymmetry, 1999, 10, 1315-1320.
DOI: 10.1016/S0957-4166(99)00097-X
26 Directed Dihydroxylation of Allylic Trichloroacetamides
Timothy J. Donohoe, Kevin Blades, Peter R. Moore, Jonathan J. G. Winter, Madeleine Helliwell and Geoffrey Stemp
J. Org. Chem. 1999, 64, 2980-2981.
DOI: 10.1021/jo982468e
25 Stereoselectivity in the double reductive alkylation of pyrroles: synthesis of cis-3,4-disubstituted pyrrolidines
Timothy J. Donohoe, Rakesh R. Harji and Rick P. C. Cousins
J. Chem Soc., Chem. Commun. 1999, 141-142.
DOI: 10.1039/A809193E
24 The Stereoselective Birch Reduction of Pyrroles
Timothy J. Donohoe, P. M. Guyo and M. Helliwell
Tetrahedron Letters, 1999, 40, 435-438.
DOI: 10.1016/S0040-4039(98)02395-8
23 Synthesis of Amino-Sugars Using the Directed Dihydroxylation Reaction
Timothy J. Donohoe, Kevin Blades and Madeleine Helliwell
J. Chem Soc., Chem. Commun. 1999, 1733-1734.
DOI: 10.1039/A904991F
22 The Synthesis of (+)-pericosine B
Timothy J. Donohoe, K. Blades, M. Helliwell, M. J. Waring, and N. J. Newcombe
Tetrahedron Lett. 1998, 39, 8755-8758.
DOI: 10.1016/S0040-4039(98)01989-3
21 The Birch Reduction of 3-Substituted Pyrroles
Timothy J. Donohoe, P. M. Guyo, R. R. Harji and R. P. C. Cousins
Tetrahedron Lett. 1998, 39, 3075-3078.
DOI: 10.1016/S0040-4039(98)00362-1
20 Stereoselectivity in the Birch Reduction of 2-Furoic Acid Derivatives
Timothy J. Donohoe, M. Helliwell, C. A. Stevenson and T. Ladduwahetty
Tetrahedron Lett., 1998, 39, 3071-3074.
DOI: 10.1016/S0040-4039(98)00361-X
19 Applications of Stoichiometric Organotransition Metal Complexes in Organic Synthesis
Timothy J. Donohoe, Rakesh R. Harji, Peter R. Moore and Michael J. Waring
J. Chem. Soc. Perkin Transactions 1, 1998, 819-859. (Review)
DOI: 10.1039/A706382B
18 The Reduction of Electron-Deficient Pyrroles Using Group I and II Metals in Ammonia
Timothy J. Donohoe, P. M. Guyo, R. L. Beddoes and M. Helliwell
J. Chem. Soc. Perkin Transactions 1, 1998, 667-672.
DOI: 10.1039/A707661D
17 The Directed Dihydroxylation of Allylic Alcohols
Timothy J. Donohoe, Peter R. Moore, Michael J. Waring and Nicholas J. Newcombe
Tetrahedron Lett. 1997, 38, 5027-5030.
DOI: 10.1016/S0040-4039(97)01061-7
16 Applications of Stoichiometric Organotransition Metal Complexes in Organic Synthesis
Timothy J. Donohoe, Paul M. Guyo, Peter R. Moore and Clare A. Stevenson
Cont. Org. Syn. 1997, 4, 22-39. (Review)
DOI: 10.1039/CO9970400022
15 Studies on the Role of Conformation and of Hydrogen-Bonding on the Dihydroxylation of Cyclic Allylic Alcohols: Application to the Synthesis of Conduritol D
Timothy J. Donohoe, Peter R. Moore and Roy L. Beddoes
J. Chem. Soc. Perkin Transactions 1, 1997, 43-51.
DOI: 10.1039/A604052G
14 Birch Reduction of Electron-Deficient Pyrroles
Timothy J. Donohoe and Paul M. Guyo
J. Org. Chem. 1996, 61, 7664-7665.
DOI: 10.1021/jo961688u
13 On the Dihydroxylation of Cyclic Allylic Alcohols
Timothy J. Donohoe, Rina Garg and Peter R. Moore
Tetrahedron Lett. 1996, 37, 3407-3410.
DOI: 10.1016/0040-4039(96)00558-8
12 Prospects for Stereocontrol in the Reduction of Aromatic Compounds
Timothy J. Donohoe, Rina Garg and Clare A. Stevenson
Tetrahedron: Asymmetry, 1996, 7, 317-344. (Review)
DOI: 10.1016/0957-4166(96)00001-8
11 Stoichiometric Organotransition Metals in Organic Synthesis
Timothy J. Donohoe
Cont. Org. Syn. 1996, 3, 1-18. (Review)
DOI: 10.1039/CO9960300001
Publications
10 Taxane Diterpenes 3: Formation of the Eight-Membered B-Ring by Semi-Pinacol Rearrangement
Philip Magnus, Louis Diorazio, Timothy J. Donohoe, Melvyn Giles, Philip Pye, James Tarrant and Stephen Thom
Tetrahedron, 1996, 52, 14147-14176.
DOI: 10.1016/0040-4020(96)00865-4
9 Taxane Diterpenes 2: Synthesis of the 7-Deoxy ABC Taxane Skeleton, and Reactions of the A-Ring
Philip Magnus, John Booth, Louis Diorazio, Timothy J. Donohoe, Vince Lynch, Nicholas Magnus, José Mendoza, Philip Pye and James G. Tarrant
Tetrahedron, 1996, 52, 14103-14146.
DOI: 10.1016/0040-4020(96)00866-6
8 Taxane Diterpenes 1: Control of Relative and Absolute Stereochemistry in Intramolecular Pyrylium Ylide-Alkene Cyclizations for the Synthesis of Taxol Precursors
William E. Bauta, John Booth, Mary E. Bos, Mark DeLuca, Louis Diorazio, Timothy J. Donohoe, Christopher Frost, Nicholas Magnus, Philip Magnus, José Mendoza, Philip Pye, James G. Tarrant, Stephen Thom and Feroze Ujjainwalla
Tetrahedron, 1996, 52, 14081-14102.
DOI: 10.1016/0040-4020(96)00867-8
7 New Strategy for the Synthesis of the Taxane Diterpenes: Formation of the Eight-membered B-ring of Taxol by Semi-pinacol Rearrangement
P. Magnus, L. Diorazio, T. J. Donohoe, M. Giles, P. Pye, J. Tarrant and S. Thom
J. Chem. Soc., Chem. Commun. 1995, 1935-1936.
DOI: 10.1039/C39950001935
6 New strategy for the synthesis of the taxane diterpenes: Formation of the BC-rings of taxol via a [5+2]-pyrylium ylide-alkene cyclization, ring expansion strategy
W. Bauta, J. Booth, M. Bos, M. DeLuca, L. Diorazio, T. J. Donohoe, N. Magnus, P. Magnus, J. Mendoza, P. Pye, J. Tarrant, S. Thom and F. Ujjainwalla
Tetrahedron Lett. 1995, 36, 5327-5330.
DOI: 10.1016/0040-4039(95)01083-T
5 Arene Chromium Tricarbonyl Stabilised Benzylic Carbocations
Stephen G. Davies and Timothy J. Donohoe
Synlett, 1993, 323-332.
DOI: 10.1055/s-1993-22443
4 Asymmetric Synthesis via Homochiral o-Anisaldehyde Chromium Tricarbonyl
Stephen G. Davies and Timothy J. Donohoe, H. Werner, A. G. Griesbeck, W. Adam, G. Bringmann, and W. Kiefer (Eds)
Selective Reactions of Metal-Activated Molecules, Vieweg, Braunschweig 1992, 9-17. (Review)
DOI: 10.1016/S0957-4166(00)82002-9
3 Stereoselective Manipulation of Acetals Derived from o-Substituted Benzaldehyde Chromium Tricarbonyl Complexes
Stephen G. Davies, Timothy J. Donohoe and J. M. J. Williams
Pure and Appl. Chem. 1992, 64, 379-386. (Review)
DOI: 10.1351/pac199264030379
2 Asymmetric Synthesis of 2-Aryl-Tetrahydropyrans via Arene Chromium Tricarbonyl Methodology 2: 2-Aryl-3-Ethyl-4-Chloro-Tetrahydropyran
Stephen. G. Davies, Timothy J. Donohoe and M. Anne Lister
Tetrahedron: Asymmetry, 1991, 2, 1089-1092.
DOI: 10.1016/S0957-4166(00)82003-0
1 Asymmetric Synthesis of 2-Aryl-Tetrahydropyrans via Arene Chromium Tricarbonyl Methodology 1: cis-2-Aryl-4-Chloro-Tetrahydropyrans
Stephen G. Davies, Timothy J. Donohoe and M. Anne Lister
Tetrahedron: Asymmetry, 1991, 2, 1085-1088.
DOI: 10.1016/S0957-4166(00)82002-9